
If the heat of vaporization of water at 100☌ is 539 calories, then subtracting the 41 calorie work component suggests that the actual binding energy of the water molecules at 100☌ is 539-41=498 calories. In the process of vaporization of water, a large amount of energy must be added to overcome the remaining cohesive forces between the molecules and an additional amount of energy goes into PdV work to expand the gas from its very small liquid volume to the volume occupied by the resulting vapor. This weakening of the intermolecular forces manifests itself in the reduction of the surface tension of water as it is heated. The remainder of the energy goes into weakening the attractive forces between the water molecules. The net gain in kinetic energy is then 16.7 calories/gram when the water is heated from 0 to 100 C. To assess the amount added to kinetic energy, the molecular speeds at the two temperatures may be evaluated with the Boltzmann speed distribution. Part of that energy increases the kinetic energy of the molecules, and some adds to the potential energy.

In the process of heating water from 0 to 100 C, 100 calories of energy must be added. Some energy details related to heating water So the absolute temperature is actually proportional to the translational kinetic energy of the molecules, while the Celsius temperatures are just chosen for convenience. But the kinetic temperature is inherently the absolute temperature, so that the ratio of the heights of the blocks is 373K/273K. From the definition of kinetic temperature, the size of the block is seen to be proportional to temperature, and the ratios of the heights of the KE blocks is the ratio of the temperatures. The sizes of the blocks which represent the kinetic energy of the molecules at 0☌ and 100☌ provide a visual illustration of the meaning of temperature and the nature of the absolute or Kelvin temperature scale. It is known that 100 calories of energy must be added to raise the temperature of one gram of water from 0° to 100☌. A bound particle at rest then has negative potential energy.
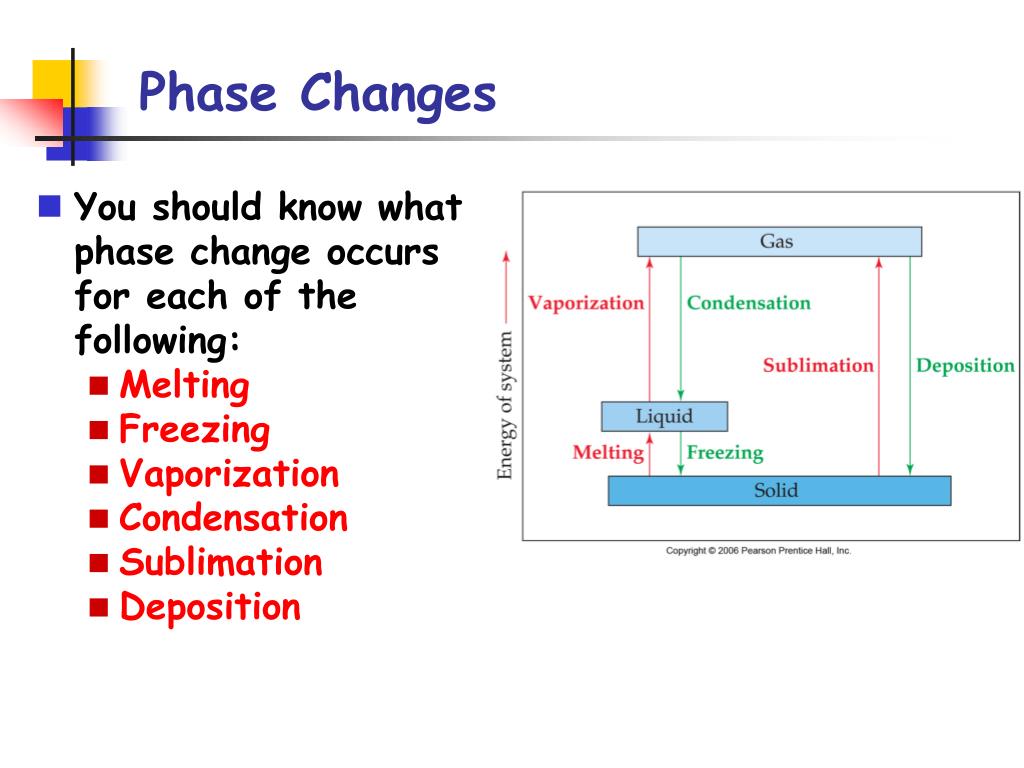
#Phase changes free
You are always free to choose the zero of potential energy, and it seems logical to choose the zero of potential energy such that a free molecule at rest has zero energy. An analogy with a mechanical system with gravitational potential energy and kinetic energy might be helpful in understanding the logic of a negative energy quantity. In discussing the energy of the phase changes in water, we found that the potential energy is treated as a negative quantity.
The data for the vaporization phase change presumes that the pressure is one standard atmosphere. The graph below presumes that the pressure is one standard atmosphere.Įnergy Involved in the Phase Changes of Water If heat were addedat a constant rate to a mass of ice to take it through its phase changes toliquid water and then to steam, the energies required to accomplish the phase changes (called the latent heat of fusion and latent heat of vaporization ) would lead to plateaus in the temperature vs time graph. \).Transitions between solid, liquid, and gaseous phases typically involvelarge amounts of energy compared to the specific heat.


 0 kommentar(er)
0 kommentar(er)
